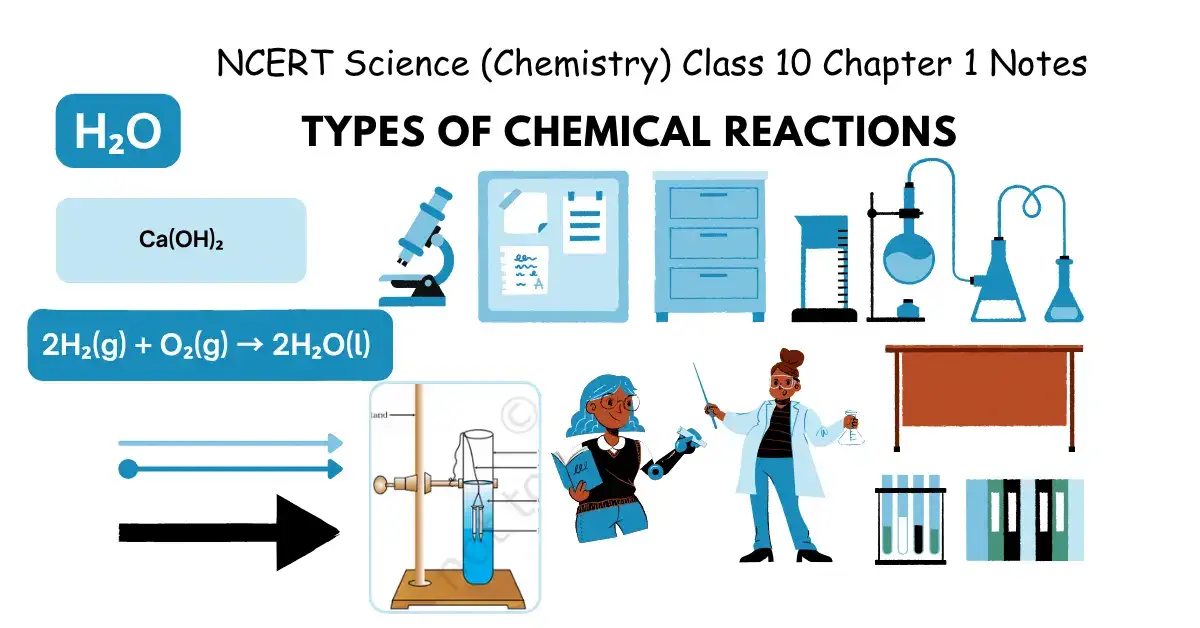
NCERT Science (Chemistry) Class 10 Chapter 1 Notes | Types Of Chemical Reactions
Topic & sub-topics covered: “Types Of Chemical Reactions, and Activities 1.4, 1.5, 1.6, 1.7, 1.8, 1.9, 1.10 & 1.11” and MCQs Questions: Chemical Reactions and Equations (All single detail notes are exam-oriented).
We have discussed in-depth and exam-oriented pointers that can be asked in the board exam of class 10th about “Types Of Chemical Reactions” from the NCERT Science (Chemistry) Class 10 Chapter 1 “Chemical Reactions and Equations“.
Download the NCERT Science (Chemistry) Class 10 Chapter 1 Chemical Reactions and Equations PDF Notes
By downloading this chapter notes PDF you will be able to access all the topics and subtopics that are related to this chapter called Chemical Reactions and Equations. This chapter have a good weightage for exam and it contains at least approx. 4 marks. So download NCERT Science (Chemistry) chapter 1 Chemical Reactions and Equations notes PDF.
Types Of Chemical Reactions
1. General Insights on Chemical Reactions:
- In chemical reactions:
a. Atoms of one element do not change into atoms of another element.
b. Atoms do not disappear or appear from elsewhere.
c. Reactions involve breaking and forming bonds between atoms to produce new substances. - The study of bond types will be covered in Chapters 3 and 4.
A. Combination Reactions
1. Definition:
A combination reaction is when two or more reactants combine to form a single product.
Activity 1.4: Reaction of calcium oxide (quick lime) with water
1. Observation:
- Heat is released, making the beaker warm.
2. Equation:
- CaO(s) + H₂O(l) → Ca(OH)₂(aq) + Heat
(Quick lime) (Slaked lime)
3. Applications of Slaked Lime:
- Used for whitewashing walls.
- Reaction with CO₂ in air forms a thin, shiny layer of calcium carbonate:
a. Ca(OH)₂(aq) + CO₂(g) → CaCO₃(s) + H₂O(l)
b. Calcium carbonate gives a glossy finish to walls.
c. Marble (CaCO₃) has the same chemical formula.
Examples of Combination Reactions
1. Burning of Coal:
- C(s) + O₂(g) → CO₂(g)
(Carbon reacts with oxygen to form carbon dioxide.)
2. Formation of Water:
- 2H₂(g) + O₂(g) → 2H₂O(l)
(Hydrogen combines with oxygen to form water.) - General Rule: Two or more substances (elements or compounds) combining to form a single product typifies a combination reaction.
Exothermic Reactions
1. Definition:
- Reactions that release heat along with product formation.
2. Characteristics:
- Heat is evolved, making the reaction mixture warm.
- Combination reactions can also be exothermic.
3. Examples of Exothermic Reactions:
- Burning of Natural Gas: CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g) + Heat
- Respiration: C₆H₁₂O₆(aq) + 6O₂(aq) → 6CO₂(aq) + 6H₂O(l) + Energy
a. Glucose reacts with oxygen in cells to release energy.
b. Provides energy for survival and is studied as respiration in Chapter 6.
4. Decomposition of Vegetable Matter:
- Produces compost and releases heat.
B. Decomposition Reaction
1. Definition of Decomposition Reaction:
- Concept: A decomposition reaction involves a single reactant breaking down into two or more simpler products.
Example: 2FeSO₄ (s) → Heat → Fe₂O₃ (s) + SO₂ (g) + SO₃ (g)
Activity 1.5: Decomposition of Ferrous Sulphate
1. Materials Used:
- 2 g of ferrous sulphate crystals (FeSO₄·7H₂O)
- Dry boiling tube.
- Burner or spirit lamp.
2. Procedure:
- Place the ferrous sulphate crystals in a dry boiling tube.
- Observe and note the green color of the crystals before heating.
- Heat the boiling tube over the flame.
- Observe the color change of the crystals and note any odour produced.
3. Observations:
- Before heating: Green colour of ferrous sulphate crystals.
- After heating:
a. The crystals lose water and change color.
b. A characteristic odour of burning Sulphur is observed.
4. Chemical Equation:
- 2FeSO₄ (s) → Heat → Fe₂O₃ (s) + SO₂ (g) + SO₃ (g)
- Products:
a. Fe₂O₃: Ferric oxide (solid).
b. SO₂ & SO₃: Sulphur dioxide and Sulphur trioxide (gases).
Example: Decomposition of Calcium Carbonate
1. Reaction:
- CaCO₃ (s) → Heat → CaO (s) + CO₂ (g)
- Products:
a. Calcium oxide (CaO, also known as quick lime).
b. Carbon dioxide ( CO₂, gas).
2. Applications:
- Calcium oxide (CaO) is widely used in industries such as cement manufacturing.
Key Exam Concepts:
1. Thermal Decomposition:
- Decomposition reactions that occur due to heating are called thermal decomposition reactions.
- Examples:
a. 2FeSO₄ (s) → Heat → Fe₂O₃ (s) + SO₂ (g) + SO₃ (g)
b. CaCO₃ (s) → Heat → CaO (s) + CO₂ (g)
2. Observational Skills:
- Color changes in reactants (e.g., green ferrous sulphate turning brown).
- Formation of gaseous products (e.g., SO₂, SO₃, CO₂)
- Characteristic odours (e.g., burning sulphur).
Exam-Oriented Questions
- Define decomposition reaction with an example.
- What is thermal decomposition? Provide an example.
- Describe the decomposition of ferrous sulphate crystals, including observations and chemical equation.
- What are the uses of quick lime (CaO) produced in the decomposition of calcium carbonate?
- Write the balanced chemical equations for:
a. Decomposition of ferrous sulphate.
b. Decomposition of calcium carbonate.
Activity 1.6: Decomposition of Lead Nitrate
1. Activity Overview:
- Objective: To observe the decomposition of lead nitrate upon heating.
2. Materials Required:
- 2 g of lead nitrate (Pb(NO₃)₂) powder.
- Boiling tube.
- Pair of tongs.
- Flame source (e.g., burner).
3. Procedure:
- Place 2 g of lead nitrate powder in a boiling tube.
- Hold the boiling tube with a pair of tongs.
- Heat the boiling tube gently over a flame.
4. Observations:
- Emission of Brown Fumes:
a. Brown fumes are emitted during the reaction.
b. These fumes are identified as nitrogen dioxide NO₂. - Color Change in Residue:
The solid residue left behind is lead oxide (PbO), which has a yellow color.
5. Reaction Details:
- Chemical Reaction: 2Pb(NO₃)₂ (s) → Heat → 2PbO(s) + 4NO₂(g) + O₂(g)
- Reaction Type:
a. Decomposition Reaction: A single reactant breaks down into multiple simpler products.
b. Thermal Decomposition: Heat is used to drive the reaction. - Products of the Reaction:
a. PbO: Yellow solid (lead oxide).
b. NO₂: Brown fumes (nitrogen dioxide).
c. O₂: Colorless gas (oxygen).
6. Key Concepts and Applications:
- Definition: A decomposition reaction is a chemical reaction where one compound breaks down into two or more simpler substances.
- Industrial Application: Decomposition reactions are used in gas production and oxide preparation.
Possible Exam Questions
- What type of reaction is the decomposition of lead nitrate?
- Write the balanced chemical equation for the thermal decomposition of lead nitrate.
- Explain the observations made when lead nitrate is heated.
- Describe the products of the decomposition reaction of lead nitrate.
- How can you identify nitrogen dioxide (NO₂) gas in the reaction?
Activity 1.7: Electrolysis of Water
1. Activity Setup:
- Objective: To study the electrolysis of water and identify the gases evolved.
2. Materials Required:
- Plastic mug.
- Rubber stoppers.
- Carbon electrodes.
- 6-volt battery.
- Dilute sulphuric acid.
- Two test tubes filled with water.
3. Procedure:
- Drill two holes at the base of a plastic mug and fit rubber stoppers in them.
- Insert carbon electrodes into the rubber stoppers.
- Connect the electrodes to a 6-volt battery.
- Fill the mug with water and add a few drops of dilute sulphuric acid.
- Place two inverted, water-filled test tubes over the electrodes.
- Switch on the battery and observe the setup.
4. Observations:
- Formation of Bubbles: Bubbles form at both electrodes, displacing water in the test tubes.
- Volume of Gases: The volume of gas collected at one electrode is double the volume collected at the other.
- Identification of Gases:
a. Larger volume gas (H₂): Produces a ‘pop’ sound when a burning candle is brought near it.
b. Smaller volume gas (O₂): Supports combustion and relights a glowing candle.
5. Chemical Reaction:
- Balanced Reaction for Electrolysis of Water:
2H₂O (l) → Electrolysis → 2H₂ (g) + O₂ (g) - Reaction Explanation:
a. At the cathode (negative electrode): 2H⁺ (aq) + 2e⁻ → H₂ (g)
b. At the anode (positive electrode): 2H₂O (l) → O₂ (g) + 4H⁺ (aq) + 4e⁻
6. Key Concepts:
- Role of Dilute Sulphuric Acid: Acts as an electrolyte to facilitate the flow of electric current.
- Gas Ratio: Hydrogen and oxygen are produced in a 2:1 volume ratio.
- Safety Precaution: Testing gases should be done with extreme caution to avoid accidents.
7. Applications of Electrolysis:
- Industrial Uses:
a. Production of hydrogen and oxygen gases.
b. Electroplating and refining of metals.
Possible Exam Questions
- What is the ratio of hydrogen to oxygen in the electrolysis of water?
- Write the balanced chemical equation for the electrolysis of water.
- Explain the process of electrolysis of water with a neat diagram.
- Why is dilute sulphuric acid added to water during electrolysis?
Activity 1.8: Decomposition of Silver Chloride
1. Activity Setup:
- Objective: To observe the decomposition of silver chloride in sunlight and understand the nature of endothermic reactions.
2. Materials Required:
- 2g of silver chloride (AgCl).
- A China dish.
- Sunlight.
3. Procedure:
- Take 2 g of white silver chloride in a China dish.
- Expose the China dish to sunlight for a certain period.
- Observe the change in color of silver chloride.
4. Observations:
- Initial Color: Silver chloride (AgCl) is white before exposure to sunlight.
- Color Change: The white silver chloride turns grey upon exposure to sunlight.
5. Chemical Reactions:
- Reaction for Decomposition of Silver Chloride:
2AgCl (s) → Sunlight → 2Ag (s) + Cl₂ (g) - Reaction for Decomposition of Silver Bromide:
2AgBr (s) → Sunlight → 2Ag (s) + Br₂ (g) - Energy Source: Light energy (from sunlight) causes the decomposition of AgCl and AgBr.
6. Key Concepts:
- Photochemical Reaction: Decomposition of silver chloride is a photochemical reaction, as it is initiated by light energy.
- Endothermic Nature: Energy from sunlight is absorbed, classifying this as an endothermic reaction.
- Similar Behavior of Silver Bromide: Silver bromide (AgBr) decomposes in a similar manner to silver chloride.
7. Applications:
- Photography:
a. The decomposition of silver halides (AgCl and AgBr) is the basis of black-and-white photography.
b. Light exposure breaks down the silver halides, creating photographic images.
Possible Exam Questions
- What happens to silver chloride when exposed to sunlight?
- Write the balanced chemical equation for the decomposition of silver chloride.
- Explain the role of light energy in the decomposition of silver chloride and silver bromide.
- What are endothermic reactions? Provide examples from the decomposition of silver halides.
C. Displacement Reaction
1. General Concept of Displacement Reactions:
- Definition: A displacement reaction occurs when a more reactive metal displaces a less reactive metal from its compound.
Activity 1.9: Reaction of Iron with Copper Sulphate
1. Materials Used:
- Three iron nails (cleaned with sandpaper).
- Two test tubes (A and B).
- 10 mL of copper sulphate solution in each test tube.
2. Procedure:
- Tie two iron nails with a thread and immerse them in test tube B containing copper sulphate solution for 20 minutes.
- Keep one iron nail aside for comparison.
- After 20 minutes, observe the changes in the copper sulphate solution and the iron nails.
3. Observations:
- The blue color of the copper sulphate solution fades in test tube B.
- The iron nails immersed in the solution develop a brownish layer.
- No changes are observed in test tube A or the iron nail kept aside.
4. Explanation:
- Iron reacts with copper sulphate, displacing copper to form iron sulphate.
- The reaction: Fe(s) + CuSO₄(aq) → FeSO₄(aq) + Cu(s)
- The brownish color on the iron nails is due to deposited copper.
- The blue color fades because copper ions are removed from the solution.
5. Examples of Displacement Reactions
- Reaction of Zinc with Copper Sulphate: Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s)
Zinc displaces copper, forming zinc sulphate and depositing copper. - Reaction of Lead with Copper Chloride: Pb(s) + CuCl₂(aq) → PbCl₂(aq) + Cu(s)
Lead displaces copper, forming lead chloride and depositing copper.
Reactivity and Displacement
1. Reactivity Series:
- Metals higher in the reactivity series displace metals lower in the series from their compounds.
Examples: Zinc and lead are more reactive than copper, allowing them to displace copper from its compounds.
2. Key Exam Question:
- Why does the blue color of copper sulphate fade in Activity 1.9?
Answer: The blue color fades because iron displaces copper from copper sulphate, forming colorless iron sulphate.
Exam Tips
- Always write the balanced chemical equation when explaining displacement reactions.
- Include observations and explanations for experimental setups to demonstrate understanding of practical applications.
- Highlight the importance of the reactivity series in predicting displacement reactions.
D. Double Displacement Reaction
Activity 1.10: Double Displacement Reaction
1. Objective:
- To observe a double displacement reaction and identify the formation of a precipitate.
2. Materials Required:
- 3 mL sodium sulphate (Na₂SO₄) solution.
- 3 mL barium chloride (BaCl₂) solution.
- Two test tubes.
3. Procedure:
- Take 3 mL of sodium sulphate solution in a test tube.
- Take 3 mL of barium chloride solution in another test tube.
- Mix the two solutions.
4. Observations:
- Formation of Precipitate:
a. A white, insoluble substance forms upon mixing the two solutions.
b. This substance is identified as barium sulphate (BaSO₄).
5. Reaction Details:
- Chemical Equation: Na₂SO₄ (aq) + BaCl₂ (aq) → BaSO₄ (s) + 2NaCl (aq)
- Products Formed:
a. Barium sulphate (BaSO₄): White precipitate.
b. Sodium chloride (NaCl): Remains dissolved in the solution. - Mechanism:
a. The SO₄²⁻ ions from sodium sulphate react with Ba²⁺ ions from barium chloride to form insoluble barium sulphate.
b. This process involves an exchange of ions, characteristic of a double displacement reaction.
6. Key Concepts:
- Double Displacement Reaction:
a. A reaction where two compounds exchange their ions to form two new compounds.
Example: Na₂SO₄ and BaCl₂ exchange ions to form BaSO₄ and NaCl. - Precipitation Reaction:
a. Any reaction resulting in the formation of an insoluble solid (precipitate).
b. In this activity, BaSO₄ is the precipitate.
7. Applications:
- Industrial Use: Precipitation reactions are used in water purification, salt production, and qualitative chemical analysis.
- Analytical Chemistry: Double displacement reactions help in detecting the presence of specific ions in a solution.
Possible Exam Questions
- What is a double displacement reaction? Provide an example.
- Write the balanced chemical equation for the reaction between sodium sulphate and barium chloride.
- Describe the steps and observations of Activity 1.10.
- Explain the mechanism of a precipitation reaction with reference to BaSO₄.
E. Oxidation and Reduction (Redox Reactions)
Key Concepts:
1. Oxidation:
- A substance is said to be oxidised if it:
a. Gains oxygen, or
b. Loses hydrogen.
2. Reduction:
- A substance is said to be reduced if it:
a. Loses oxygen, or
b. Gains hydrogen.
3. Redox Reaction:
- A reaction in which one reactant is oxidised, and the other is reduced simultaneously.
- Examples:
a. Copper and Oxygen Reaction (2Cu + O₂ → 2CuO):
i. Copper is oxidised to copper(II) oxide.
b. Copper Oxide and Hydrogen Reaction (CuO + H₂ → Cu + H₂O):
i. Copper(II) oxide is reduced to copper.
ii. Hydrogen is oxidised to water.
4. Chemical Equations with Explanation:
- Formation of Copper Oxide: 2Cu + O₂ → Heat → 2CuO
a. Oxidation: Copper gains oxygen to form black copper(II) oxide (CuO). - Reduction of Copper Oxide: CuO + H₂ → Heat → Cu + H₂O
- a. Reduction: Copper(II) oxide loses oxygen to form copper.
b. Oxidation: Hydrogen gains oxygen to form water. - Other Examples of Redox Reactions:
a. Reaction 1: ZnO + C → Zn + CO
i. Zinc oxide (ZnO) is reduced to zinc (Zn).
ii. Carbon (C) is oxidised to carbon monoxide (CO).
b. Reaction 2: MnO₂ + 4HCl → MnCl₂ + 2H₂O + Cl₂
i. Manganese dioxide (MnO₂) is reduced to manganese(II) chloride (MnCl₂).
ii. Hydrochloric acid (HCl) is oxidised to chlorine gas (Cl₂).
5. Key Observations:
- Color Change in Copper Reaction:
a. Copper (Cu) turns black upon oxidation (CuO).
b. Black copper(II) oxide (CuO) turns brown when reduced back to copper (Cu). - Oxygen and Hydrogen Transfer:
a. Oxidation involves oxygen gain or hydrogen loss.
b. Reduction involves oxygen loss or hydrogen gain.
6. Applications of Redox Reactions:
- Industrial Uses:
a. Metal extraction (e.g., zinc from zinc oxide).
b. Production of chemicals (e.g., chlorine gas from HCl). - Daily Life:
a. Corrosion of metals.
b. Respiration and combustion processes.
Possible Exam Questions
- Define oxidation and reduction with examples.
- Write the balanced chemical equation for the reaction between CuO and H₂.
- Explain redox reactions with the example of copper and oxygen reaction.
- Discuss the role of oxidation and reduction in the reaction between MnO₂ and HCl.
Next & Previous Topics of NCERT/CBSE Science (Chemistry) Class 10 Chapter 1: Chemical Reactions and Equations
| Topics No. | Topics Name |
|---|---|
| 1 | Chemical Equations |
| 2 | Types Of Chemical Reactions |
| 3 | Have You Observed The Effects Of Oxidation Reactions In Everyday Life? |
MCQs on NCERT Science (Chemistry) Class 10 Chapter 1 Topic – Types Of Chemical Reactions
Here are the top exam-oriented MCQ-type questions on “Types Of Chemical Reactions” that you should prepare for your CBSE or state board exams:
Question 1. What is a combination reaction?
a) A reaction where one reactant breaks into simpler products
b) A reaction where two or more reactants form a single product
c) A reaction where ions are exchanged between reactants
d) A reaction involving displacement of an element
Answer: b) A reaction where two or more reactants form a single product
Question 2. What is the product of the reaction CaO (s) + H₂O (l) → Ca(OH)₂ (aq) + Heat?
a) Quick lime
b) Calcium carbonate
c) Slaked lime
d) Carbon dioxide
Answer: c) Slaked lime
Question 3. Which chemical reaction is used in whitewashing walls?
a) CaCO₃ → CaO + CO₂
b) Ca(OH)₂ + CO₂ → CaCO₃ + H₂O
c) C + O₂ → CO₂
d) Zn + CuSO₄ → ZnSO₄ + Cu
Answer: b) Ca(OH)₂ + CO₂ → CaCO₃ + H₂O
Question 4. What type of reaction occurs when coal burns in oxygen?
a) Decomposition reaction
b) Combination reaction
c) Displacement reaction
d) Precipitation reaction
Answer: b) Combination reaction
Question 5. Which of the following is an exothermic reaction?
a) Electrolysis of water
b) Respiration
c) Decomposition of silver chloride
d) Decomposition of calcium carbonate
Answer: b) Respiration
Question 6. Which gas is evolved when ferrous sulphate is decomposed by heat?
a) Oxygen and nitrogen
b) Sulphur dioxide and sulphur trioxide
c) Hydrogen and oxygen
d) Nitrogen dioxide
Answer: b) Sulphur dioxide and sulphur trioxide
Question 7. What is the color change observed when ferrous sulphate is heated?
a) Green to black
b) Green to yellow
c) Black to white
d) Green to blue
Answer: a) Green to black
Question 8. Which type of energy causes decomposition of silver chloride?
a) Heat
b) Light
c) Electricity
d) Pressure
Answer: b) Light
Question 9. The decomposition of calcium carbonate produces:
a) Carbon dioxide and calcium hydroxide
b) Calcium oxide and carbon dioxide
c) Calcium hydroxide and carbon monoxide
d) Quick lime and water
Answer: b) Calcium oxide and carbon dioxide
Question 10. Which reaction is used in black and white photography?
a) Thermal decomposition of lead nitrate
b) Electrolysis of water
c) Decomposition of silver chloride by sunlight
d) Combination reaction of calcium oxide with water
Answer: c) Decomposition of silver chloride by sunlight
Question 11. What happens when an iron nail is immersed in copper sulphate solution?
a) Iron is deposited on the nail
b) Copper is deposited on the nail
c) Blue color of the solution intensifies
d) No change occurs
Answer: b) Copper is deposited on the nail
Question 12. Which of the following represents a displacement reaction?
a) Zn + CuSO₄ → ZnSO₄ + Cu
b) CaCO₃ → CaO + CO₂
c) H₂ + O₂ → H₂O
d) CaO + H₂O → Ca(OH)₂
Answer: a) Zn + CuSO₄ → ZnSO₄ + Cu
Question 13. In the reaction Fe + CuSO₄ → FeSO₄ + Cu, which element is displaced?
a) Iron
b) Copper
c) Sulphur
d) Oxygen
Answer: b) Copper
Question 14. What type of reaction occurs between sodium sulphate and barium chloride?
a) Displacement reaction
b) Decomposition reaction
c) Combination reaction
d) Double displacement reaction
Answer: d) Double displacement reaction
Question 15. The white precipitate formed in the reaction between sodium sulphate and barium chloride is:
a) Barium sulphate
b) Sodium chloride
c) Sodium sulphate
d) Barium chloride
Answer: a) Barium sulphate
Question 16. What happens to copper when it is heated in the presence of oxygen?
a) It reduces to copper(II) oxide
b) It oxidizes to copper(II) oxide
c) It combines with water to form copper hydroxide
d) It decomposes to form copper sulphate
Answer: b) It oxidizes to copper(II) oxide
Question 17. In the reaction CuO + H₂ → Cu + H₂O, hydrogen is:
a) Oxidized
b) Reduced
c) Neutralized
d) Precipitated
Answer: a) Oxidized
Question 18. Which of the following is a redox reaction?
a) CaO + H₂O → Ca(OH)₂
b) 2AgCl → 2Ag + Cl₂
c) MnO₂ + 4HCl → MnCl₂ + Cl₂ + 2H₂O
d) Na₂SO₄ + BaCl₂ → BaSO₄ + 2NaCl
Answer: c) MnO₂ + 4HCl → MnCl₂ + Cl₂ + 2H₂O
Question 19. In the reaction ZnO + C → Zn + CO, carbon acts as:
a) Oxidizing agent
b) Reducing agent
c) Catalyst
d) Precipitate
Answer: b) Reducing agent
Question 20. Which substance is oxidized in the reaction 2Cu + O₂ → 2CuO?
a) Copper
b) Oxygen
c) Hydrogen
d) Sulphur
Answer: a) Copper