NCERT Science (Chemistry) Class 10 Chapter 1 Notes | Chemical Equations
Topic & sub-topics covered: “Chemical Equations, Activities 1.1, 1.2, & 1.3, Types of Chemical Reactions” and MCQs Questions: Chemical Reactions and Equations (All single detail notes are exam-oriented).
We have discussed in-depth and exam-oriented pointers that can be asked in the board exam of class 10th about “Chemical Equations” from the NCERT Science (Chemistry) Class 10 Chapter 1 “Chemical Reactions and Equations“.
Download the NCERT Science (Chemistry) Class 10 Chapter 1 Chemical Reactions and Equations PDF Notes
By downloading this chapter notes PDF you will be able to access all the topics and subtopics that are related to this chapter called Chemical Reactions and Equations. This chapter have a good weightage for exam and it contains at least approx. 4 marks. So download NCERT Science (Chemistry) chapter 1 Chemical Reactions and Equations notes PDF.
Overview Chemical Equations
1. General Observations of Daily Life:
- Milk left at room temperature during summers undergoes a chemical change.
- Iron objects like a tawa, pan, or nail exposed to a humid atmosphere undergo rusting.
- Fermentation of grapes involves a chemical change.
- Cooking food alters the nature and composition of the ingredients.
- Digestion of food in the body is a chemical process.
- Respiration involves a chemical reaction where glucose is broken down to release energy.
2. Concept of Chemical Reactions:
- A chemical reaction occurs when the nature and identity of a substance change.
- A chemical change indicates the occurrence of a chemical reaction.
- Key indicators of chemical reactions include:
- Change in state.
- Change in color.
- Evolution of gas.
- Change in temperature.
Activity 1.1: Burning of Magnesium Ribbon
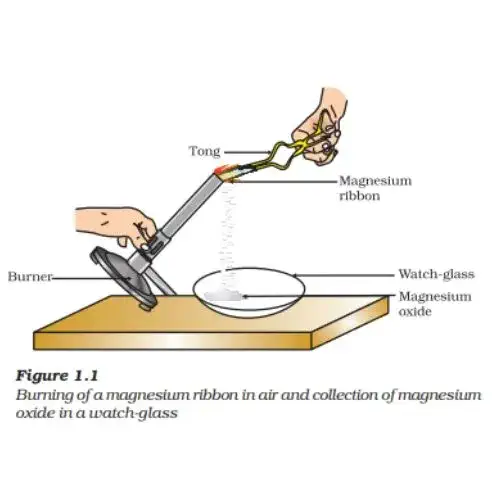
1. Procedure:
- Rub a 3-4 cm magnesium ribbon with sandpaper to clean it.
- Hold it with tongs and burn it using a spirit lamp or burner.
- Collect the ash in a watch glass.
2. Observation:
- The magnesium ribbon burns with a dazzling white flame.
- A white powder, magnesium oxide, is formed.
3. Chemical Explanation:
- The reaction involves magnesium combining with oxygen from the air to form magnesium oxide.
Activity 1.2: Reaction Between Lead Nitrate and Potassium Iodide
1. Procedure:
- Take lead nitrate solution in a test tube.
- Add potassium iodide solution to it.
2. Observation:
- A chemical reaction takes place, indicating a visible change.
Activity 1.3: Reaction Between Zinc and Acid
1. Procedure:
- Add dilute hydrochloric acid or sulfuric acid to zinc granules in a conical flask/test tube.
- Handle acids with care to avoid accidents.
2. Observations:
- A reaction occurs around the zinc granules.
- The temperature of the flask/test tube changes, indicating an exothermic reaction.
Summary of Observations
- Observations that help identify chemical reactions:
- Change in state of the substance.
- Change in color of the reactants or products.
- Gas evolution during the reaction.
- Temperature changes (heat absorption or release).
Types of Chemical Reactions
- Various types of chemical reactions occur around us daily.
- The study of chemical reactions includes:
- Symbolic representation.
- Understanding their types and occurrences.
Chemical Equations
1. Description of Chemical Reactions:
- Activity 1.1 Summary: When a magnesium ribbon burns in oxygen, it forms magnesium oxide.
- Word-Equation:
- Reactants: Magnesium + Oxygen
- Product: Magnesium oxide
- Written as: Magnesium + Oxygen → Magnesium oxide (eq. 1.1)
- Reactants and Products:
- Reactants: Substances that undergo chemical changes (e.g., magnesium and oxygen).
- Product: The new substance formed during the reaction (e.g., magnesium oxide).
2. Components of a Word-Equation:
- A word-equation represents a chemical reaction in a simplified form.
- Structure:
- Reactants: Written on the Left-Hand Side (LHS), separated by a plus sign (+).
- Products: Written on the Right-Hand Side (RHS), separated by a plus sign (+).
- Word-equations help in concise and clear representation of chemical reactions.
Writing a Chemical Equation
1. Representation of Chemical Equations:
- Alternative Representation: Chemical equations can be made more concise by using chemical formulae instead of words.
- Definition: A chemical equation represents a chemical reaction in symbolic form.
- Example:
- Word Equation: Magnesium + Oxygen → Magnesium oxide
- Chemical Equation: Mg + O₂ → MgO (eq. 1.2)
2. Balanced and Unbalanced Equations:
- Atom Counting:
- Count the number of atoms of each element on both sides of the equation.
- If the number of atoms is equal on both sides, the equation is balanced.
- If not, the equation is unbalanced.
- Unbalanced Equation:
- An unbalanced equation does not satisfy the law of conservation of mass.
- Such an equation is referred to as a skeletal chemical equation.
3. Skeletal Chemical Equation:
- Definition: A skeletal chemical equation is the unbalanced representation of a chemical reaction.
- Example: Mg + O₂ → MgO is a skeletal equation for the burning of magnesium in air.
Balanced Chemical Equations
1. Law of Conservation of Mass:
- The law of conservation of mass states that mass can neither be created nor destroyed in a chemical reaction.
- The total mass of the reactants is always equal to the total mass of the products.
- The number of atoms of each element remains unchanged before and after a chemical reaction.
2. Balancing Chemical Equations:
- Reason for Balancing: To satisfy the law of conservation of mass.
- Steps for Balancing Equations:
- Draw boxes around the chemical formulae to avoid altering them.
- Count the number of atoms for each element on both sides of the equation.
- Start with the compound that has the maximum number of atoms and balance the atoms one by one.
- Use the smallest whole-number coefficients to balance the equation.
- Verify the balance by recounting the atoms of each element on both sides.
3. Example 1: Balanced Equation for Activity 1.3
- Reaction: Zinc + Sulphuric acid → Zinc sulphate + Hydrogen
- Balanced Equation:
- Skeletal: Zn + H₂SO₄ → ZnSO₄ + H₂ (eq. 1.3)
- Balanced (Number of atoms match): Zn (1), H (2), S (1), O (4).
4. Example 2: Balancing Fe + H₂O → Fe₃O₄ + H₂ (eq. 1.4)
- Step-by-Step Process:
- Step I: Write the unbalanced equation and count atoms for each element.
- Step II: Start with oxygen in Fe₃O₄ (4 atoms) and balance using 4 H₂O molecules.
- Step III: Balance hydrogen atoms by ensuring 8 H atoms on both sides (4 H₂O → 4 H₂).
- Step IV: Balance iron by taking 3 Fe on the LHS.
- Step V: Verify the balanced equation:
3Fe + 4H₂O → Fe₃O₄ + 4H₂. (eq. 1.9) (balanced equation)
5. Adding Physical States:
- Include the physical states of substances for more detailed equations.
- Example: 3Fe(s) + 4H₂O(g) → Fe₃O₄(s) + 4H₂(g). (eq. 1.10)
6. Conditions in Chemical Equations:
- Notations for Physical States:
- (s): Solid
- (l): Liquid
- (g): Gas
- (aq): Aqueous (solution in water).
- Example:
- CO(g) + 2H₂(g) → CH₃OH(l) (Reaction conditions: 340 atm pressure). (eq. 1.11)
- 6CO₂(aq) + 12H₂O(l) + Sunlight → C₆H₁₂O₆(aq) + 6O₂(aq) + 6H₂O(l) (eq. 1.12)
(Catalyst: Chlorophyll).
7. Application to Eq. (1.2): Mg + O₂ → MgO
- Balancing Steps:
- Oxygen: 2 atoms on LHS, 1 on RHS → Place coefficient 2 on MgO.
- Magnesium: 2 atoms on RHS → Place coefficient 2 on Mg.
- Balanced Equation: 2Mg + O₂ → 2MgO.
Next & Previous Topics of NCERT/CBSE Science (Chemistry) Class 10 Chapter 1: Chemical Reactions and Equations
| Topics No. | Topics Name |
|---|---|
| 1 | Chemical Equations |
| 2 | Types Of Chemical Reactions |
| 3 | Have You Observed The Effects Of Oxidation Reactions In Everyday Life? |
MCQs on NCERT Science (Chemistry) Class 10 Chapter 1 Topic – Chemical Equations
Here are the top exam-oriented MCQ-type questions on “Chemical Equations” that you should prepare for your CBSE or state board exams:
Question 1. What is observed when magnesium ribbon is burnt in air?
a) It emits a colored flame
b) It forms a white powder
c) It emits an odor
d) It does not react
Answer: b) It forms a white powder
Question 2. The white powder formed after burning magnesium ribbon is:
a) Magnesium chloride
b) Magnesium sulphate
c) Magnesium oxide
d) Magnesium hydroxide
Answer: c) Magnesium oxide
Question 3. The reaction between magnesium and oxygen is an example of a:
a) Physical change
b) Chemical change
c) Reversible reaction
d) Decomposition reaction
Answer: b) Chemical change
Question 4. Which observation indicates a chemical reaction in Activity 1.2 (Lead nitrate + Potassium iodide)?
a) Change in temperature
b) Evolution of gas
c) Formation of a precipitate
d) No reaction
Answer: c) Formation of a precipitate
Question 5. What type of change occurs when zinc reacts with dilute hydrochloric acid?
a) Physical change
b) Chemical change
c) No change
d) Phase change
Answer: b) Chemical change
Question 6. The reaction between zinc and hydrochloric acid produces:
a) Zinc hydroxide and hydrogen
b) Zinc oxide and hydrogen
c) Zinc sulphate and water
d) Zinc chloride and hydrogen
Answer: d) Zinc chloride and hydrogen
Question 7. Which of the following is NOT a sign of a chemical reaction?
a) Change in state
b) Change in temperature
c) Change in color
d) Retention of original identity
Answer: d) Retention of original identity
Question 8. The skeletal equation for burning magnesium is:
a) Mg + H₂O → MgO + H₂
b) Mg + O₂ → MgO
c) 2Mg + O₂ → 2MgO
d) Mg + O₂ → MgO₂
Answer: b) Mg + O₂ → MgO
Question 9. What does the arrow in a chemical equation represent?
a) Addition of reactants
b) Direction of reaction
c) Removal of a product
d) Heat evolved
Answer: b) Direction of reaction
Question 10. Why do we balance a chemical equation?
a) To obey the law of constant proportion
b) To simplify the reaction
c) To comply with the law of conservation of mass
d) To ensure products are formed
Answer: c) To comply with the law of conservation of mass
Question 11. What is the physical state of water in the equation 3Fe + 4H₂O → Fe₃O₄ + 4H₂?
a) Solid
b) Liquid
c) Gas
d) Aqueous
Answer: c) Gas
Question 12. Which element should be balanced first in the reaction 3Fe + 4H₂O → Fe₃O₄ + 4H₂?
a) Iron (Fe)
b) Hydrogen (H)
c) Oxygen (O)
d) None of these
Answer: c) Oxygen (O)
Question 13. The balanced chemical equation for 3Fe + 4H₂O → Fe₃O₄ + 4H₂ is:
a) 3Fe + 4H₂O → Fe₃O₄ + 4H₂
b) Fe + H₂O → Fe₃O₄ + H₂
c) 2Fe + 3H₂O → 2FeO + 3H₂
d) 3Fe + H₂O → Fe₃O₄ + H₂
Answer: a) 3Fe + 4H₂O → Fe₃O₄ + 4H₂
Question 14. In which physical state is magnesium used in Activity 1.1?
a) Solid
b) Liquid
c) Gas
d) Aqueous
Answer: a) Solid
Question 15. Which method is commonly used to balance chemical equations?
a) Algebraic method
b) Hit-and-trial method
c) Oxidation method
d) None of these
Answer: b) Hit-and-trial method
Question 16. What does (aq) denote in a chemical equation?
a) Solid state
b) Liquid state
c) Gaseous state
d) Aqueous state
Answer: d) Aqueous state
Question 17. The reaction Zn + H₂SO₄ → ZnSO₄ + H₂ is an example of:
a) Combination reaction
b) Displacement reaction
c) Decomposition reaction
d) Redox reaction
Answer: b) Displacement reaction
Question 18. In CO (g) + 2H₂ (g) → CH₃OH (l), the conditions required are:
a) High temperature
b) High pressure
c) Catalyst presence
d) Both b and c
Answer: d) Both b and c
Question 19. What is the role of chlorophyll in photosynthesis?
a) Acts as a reactant
b) Acts as a catalyst
c) Acts as a product
d) Acts as a by-product
Answer: b) Acts as a catalyst
Question 20. What is the product formed in 6CO₂ + 12H₂O + Sunlight → C₆H₁₂O₆ + 6O₂ + 6H₂O?
a) Carbon dioxide
b) Water
c) Glucose
d) Oxygen
Answer: c) Glucose